You’ve probably heard of Hydrogen Peroxide for its’ use to whiten teeth, and disinfect cuts and surfaces. But what makes this chemical so versatile and effective?
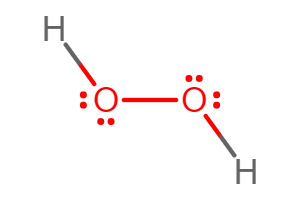
Hydrogen peroxide is a water molecule with an extra oxygen atom. It is a non-toxic, natural disinfectant, that abolishes the main components of germ cells. It can also effectively break down and eliminate viruses, fungi, and bacteria. In fact, studies have shown that this compound is more effective in killing household bacteria than most common cleaning products. Surfaces such as metal, glass, and plastic can be easily and safely disinfected with the use of the compound which significantly reduces the germs on these surfaces. The disinfectant works by releasing free oxygen radicals; which decomposes pollution. Thus, only water remains. This compound can also be used as an anti-inflammatory of the gums and skin.
In contrast to many other disinfecting agents, hydrogen peroxide doesn’t leave a residue or gas when applied. This is due to its complete water-soluble nature. The effectiveness of the disinfectant properties of hydrogen peroxide comes from its’ ability to break down bacterial cell walls through oxidation. When the compound comes in contact with a bacterial cell, it attracts electrons with the high level of reactivity of the oxygen atom; which, in turn, damages the cells.
NPure uses the oxidation and reactive properties of Hydrogen Peroxide in the PURStrike disinfecting formula disseminated by the dry fog. When the dry fog diffuses across the room and bounces off of surfaces, the agent penetrates the cells of bacterial organisms, oxidizes; thus damaging the internal structure of the cell permanently.